Detection of Remdesivir by Liquid Chromatography with Tandem Mass Spectrometry in a Patient with SARS-CoV-2 Infection
DOI:
https://doi.org/10.54779/chl20220687Keywords:
antivirotics, remdesivir, liquid chromatography, mass spectrometry, LC-MS/MSAbstract
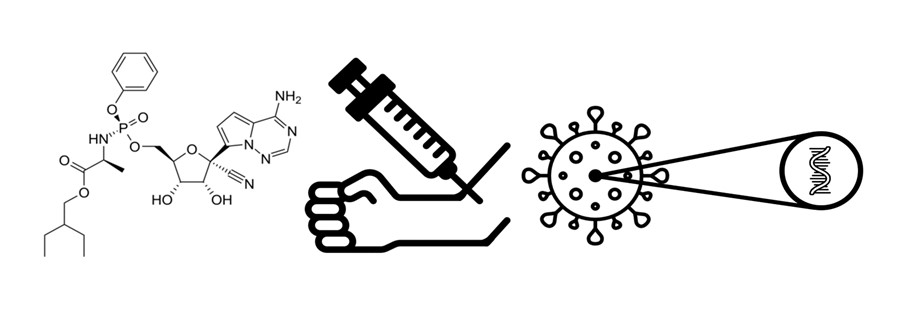
One of the officially approved medications for the treatment of the pandemic disease COVID-19, caused by the SARS-CoV-2 virus, is remdesivir. This antiviral molecule is a prodrug that is metabolized into its active form (an ATP analogue). Because of its hepatotoxicity and nephrotoxicity, it is necessary to monitor the serum concentrations of remdesivir. For the therapeutic drug monitoring of remdesivir, a method using liquid chromatography with tandem mass spectrometry (LC-MS/MS) in positive electrospray ionization mode was developed. Mass detection was done via triple quadrupole in the Multiple reaction monitoring mode. Separation was done on Zorbax C18 column at 35 °C in mobile phase gradient and flow 0.4 mL min–1 (A – 0.1% formic acid in water, B – 0.1% formic acid in 95% acetonitrile). Time of analysis was 4 minutes. LC-MS/MS method was successfully validated. Calibration was done in blood serum and plasma and it was linear in the range of tested concentrations (0–1000 ng mL–1). Samples were prepared by protein precipitation. The method was used to measure remdesivir concentration in a patient with SARS-CoV-2 infection. The measured concentration 60 minutes after remdesivir application was 175±15 ng mL–1.